[ad_1]
AP — The US is getting one other COVID-19 vaccine alternative because the Meals and Drug Administration on Wednesday cleared Novavax pictures for adults.
Novavax makes a extra conventional kind of shot than the three different COVID-19 vaccines out there to be used within the US — and one which’s already out there in Europe and a number of different nations.
Practically 1 / 4 of American adults nonetheless haven’t gotten their main vaccinations even this late within the pandemic, and consultants count on at the very least a few of them to roll up their sleeves for a extra standard possibility — a protein-based vaccine.
The Maryland firm additionally hopes its pictures can grow to be a prime booster alternative within the US and past. Tens of tens of millions of Individuals nonetheless want boosters that consultants name crucial for the absolute best safety because the coronavirus continues to mutate.
For now, the FDA licensed Novavax’s preliminary two-dose sequence for individuals 18 and older.
“I encourage anybody who’s eligible for, however has not but obtained, a COVID-19 vaccine to think about doing so,” FDA Commissioner Dr. Robert Califf mentioned in a press release.
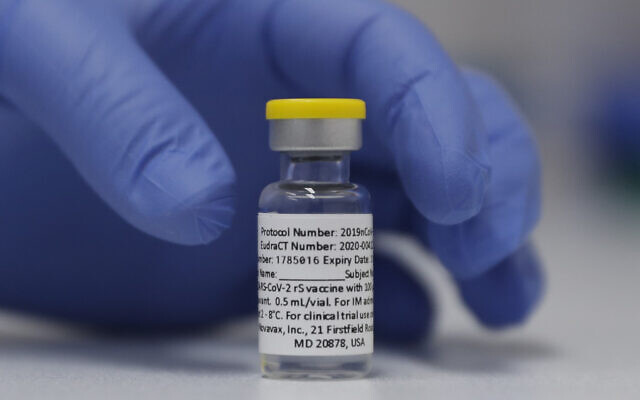
A vial of the Novavax vaccine is seen prepared to be used within the trial at St. George’s College hospital in London, October 7, 2020. (AP Photograph/Alastair Grant)
Earlier than pictures start, the Facilities for Illness Management and Prevention should suggest how they need to be used. A call is anticipated subsequent week.
Novavax CEO Stanley Erck advised The Related Press that he anticipated the US to develop use of the vaccine past unvaccinated adults pretty rapidly.
Already the FDA is evaluating it for these as younger as 12, Erck mentioned. Novavax has additionally submitted information on booster doses, together with “mix-and-match” use in individuals who’d earlier obtained Pfizer or Moderna vaccinations.
The Biden administration has purchased 3.2 million Novavax doses to date, and Erck mentioned vaccinations ought to start later this month.
Sharon Bentley of Argyle, Texas, is without doubt one of the holdouts. Bentley was hesitant concerning the first COVID-19 vaccines, however then her husband volunteered for a Novavax trial, getting two doses and later a booster.
Her husband’s constructive expertise with a extra tried-and-true expertise, “that satisfied me,” Bentley mentioned, including that she deliberate to inform some unvaccinated mates concerning the possibility too.
The Novavax vaccine is fabricated from copies of the spike protein that coats the coronavirus, packaged into nanoparticles that to the immune system resemble a virus. Then an immune-boosting ingredient, or adjuvant, that’s created from the bark of a South American tree is added that acts as a pink flag to make sure these particles look suspicious sufficient to spark a powerful immune response.
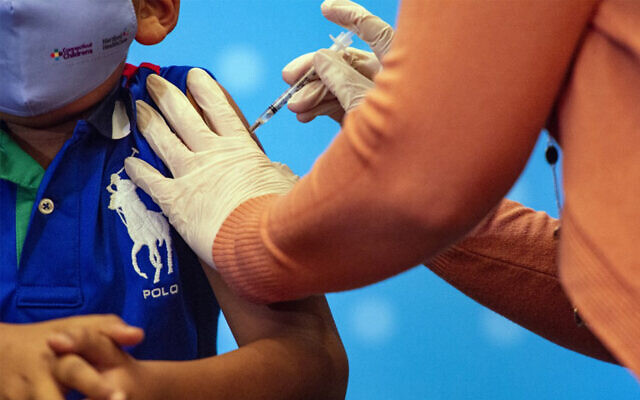
A six-year-old receives the COVID-19 vaccine in Hartford, Connecticut, November 2, 2021. (Joseph Prezioso/AFP)
Protein vaccines have been used for years to stop hepatitis B, shingles and different ailments. It’s a really totally different expertise than the dominant mRNA Pfizer and Moderna COVID-19 vaccines that ship genetic directions for the physique to supply its personal copies of the spike protein. The lesser-used Johnson & Johnson possibility makes use of a innocent chilly virus to ship spike-making directions.
Like the opposite vaccines used within the US, the Novavax pictures have proved extremely efficient at stopping COVID-19’s most extreme outcomes. Typical vaccine reactions had been delicate, together with arm ache and fatigue. However the FDA did warn about the potential for a uncommon danger, coronary heart irritation, that additionally has been seen with the Pfizer and Moderna vaccines.
The Novavax vaccine was examined lengthy earlier than the Omicron variant struck. However final month, the corporate launched information exhibiting a booster dose promised a powerful immune response even in opposition to Omicron’s latest kinfolk — preliminary proof that a number of of the FDA’s scientific advisers known as compelling.
Nonetheless, US regulators are planning for a fall booster marketing campaign utilizing Pfizer and Moderna pictures that higher goal Omicron subtypes — and Novavax additionally has begun testing up to date pictures. Erck mentioned the corporate might have up to date doses out there late within the 12 months.
European regulators not too long ago cleared the Novavax vaccine for use in kids as younger as age 12, and several other nations have licensed booster doses of its authentic vaccine.
Earlier manufacturing difficulties held up the vaccine, though Erck mentioned these have been solved and Novavax can meet world demand. A lot of the corporate’s vaccine, together with doses for the US, are being produced by the Serum Institute of India, the world’s largest vaccine producer.
[ad_2]
Source link



