[ad_1]
A COVID-19 booster focusing on two variations of the coronavirus in a single shot supplied stronger and broader safety than the present booster, which targets just one model, in response to scientific trial outcomes launched this week by vaccine maker Moderna.
The outcomes are preliminary and haven’t been peer reviewed or revealed in a scientific journal. However Moderna touted the findings as proof that bivalent or multivalent vaccines—those who goal two or extra variations of the virus in a single shot—are the way in which ahead for COVID-19 boosters.
Moderna and different vaccine makers are on a mission to develop boosters that might restore the as soon as terribly excessive ranges of safety that mRNA-based COVID-19 vaccines initially offered, whereas additionally defending in opposition to future variants. The primary-generation mRNA vaccines have been all designed to focus on the ancestral model of SARS-CoV-2 remoted in Wuhan, China—they usually did so fairly successfully, displaying efficacy in opposition to symptomatic illness within the ballpark of 95 %. However the virus has advanced into variants that may evade vaccine-derived protections. The newest variant, omicron, considerably diminished vaccine effectiveness in opposition to symptomatic illness, although safety in opposition to extreme illness stays sturdy. Booster doses of the present vaccine design buoy safety however do not restore the excessive ranges seen beforehand. And the virus continues to evolve.
As such, vaccine makers are testing variant-specific boosters in addition to mixture pictures. Moderna and Pfizer/BioNTech—joint makers of the opposite main mRNA COVID-19 vaccine—swiftly introduced plans for an omicron-specific vaccine in December, earlier than the fast-moving variant swept the globe. However thus far, early animal knowledge has advised {that a} booster dose focusing on solely the omicron variant could not supply a bonus over the present vaccines at defending in opposition to omicron. Whereas variant-specific vaccine trial knowledge continues to come back in, vaccine makers have additionally been engaged on mixture pictures. Earlier this month, as an illustration, the Nationwide Institutes of Well being introduced the beginning of a scientific trial (in collaboration with Moderna) that’s testing six totally different booster regimens, 4 of which contain a mixture shot.
Recent knowledge
Moderna now has knowledge on certainly one of its first mixture pictures, which targets the ancestral pressure plus the beta variant. The beta variant was first recognized in South Africa and dubbed a “variant of concern” in December 2020 after it demonstrated a capability to evade vaccine-derived immune responses. Although consultants initially feared it might trigger an omicron-like wave of infections, beta by no means grew to become extensively prevalent within the US and has since been fully elbowed out by omicron.
Nonetheless, vaccine makers had begun engaged on beta-targeting vaccines final yr. And that work has confirmed considerably helpful now as a result of beta shares among the key mutations for dodging protecting antibodies which might be present in omicron. Thus, mixture vaccines focusing on beta could foreshadow benefits that omicron-targeting mixture vaccines could have sooner or later.
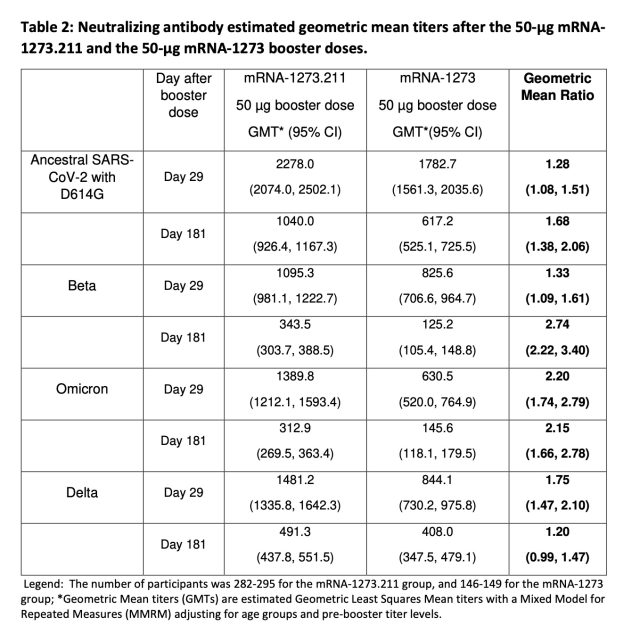
The recent knowledge launched by Moderna checked out neutralizing antibody ranges in round 300 individuals who acquired a 50-microgram dose of the beta/ancestral combo vaccine (dubbed mRNA-1273.211). Antibody ranges in that group have been in contrast with these from round 150 individuals who acquired the present 50-microgram booster (mRNA-1273) that targets the ancestral model of the virus. In contrast with the present booster, the beta/ancestral combo shot generated increased ranges of neutralizing antibodies in opposition to the ancestral virus in addition to the beta, omicron, and delta variants. Within the case of omicron, the combo shot generated neutralizing antibody ranges twice as excessive as the present shot (when evaluating geometric imply titres). That two-fold benefit was maintained after six months, as effectively. Additional, there have been no security issues with the combo vaccine through the trial and the side-effect profile regarded about the identical as that of the present booster.
Fall technique
After all, this research has limitations. The variety of folks within the trial was not enormous, and the research is just not giant sufficient to take a look at vaccine effectiveness. The research additionally doesn’t have a look at different sorts of immune responses, comparable to cell-based responses. However it did strongly counsel {that a} bivalent vaccine may out-compete the present vaccine, as a result of neutralizing antibody ranges are likely to correlate with safety. The authors of the research speculated that the extra virus targets current within the mixture vaccine induce “additional maturation and evolution” of antibody responses in vaccinated folks. “Subsequently, immunization with the first collection doesn’t set a ceiling to the neutralizing antibody response,” they wrote, “and a booster dose of the bivalent vaccine elicits a strong response with titers which might be prone to be protecting in opposition to COVID-19.”
In a press release, Moderna CEO Stéphane Bancel mentioned the findings have bolstered the corporate’s optimism for mixture pictures. “We imagine that these outcomes validate our bivalent technique, which we introduced and started pursuing in February 2021. The outcomes point out that mRNA-1273.211 [the combo shot] on the 50 µg dose stage induced increased antibody responses than the 50 µg mRNA-1273 [current] booster, even when further variants of concern weren’t included within the booster vaccine,” Bancel mentioned.
With their technique set for future boosters, Moderna expects to offer newly formulated boosters for the autumn. Nevertheless, Bancel anticipates that an omicron/ancestral combo booster will present even stronger, broader safety.
“Our newest bivalent booster candidate, mRNA-1273.214, which mixes the at the moment licensed Moderna COVID-19 booster with our omicron-specific booster candidate, stays our lead candidate for the autumn 2022 Northern Hemisphere booster,” Bancel mentioned. “We sit up for sharing preliminary knowledge on mRNA-1273.214 later within the second quarter. We imagine {that a} bivalent booster vaccine, if licensed, would create a brand new software as we proceed to answer rising variants.”
[ad_2]
Source link

